Abstract
Introduction: Daratumumab (DARA) is a human IgGκ monoclonal antibody targeting CD38 with a direct on-tumor and immunomodulatory mechanism of action. DARA is approved as monotherapy and in combination with standard-of-care regimens for patients with relapsed/refractory multiple myeloma and in combination with standard-of-care regimens for patients with NDMM. In the primary analysis of the phase 3 MAIA study (median follow-up, 28.0 months), D-Rd significantly improved progression-free survival (PFS; median not reached [NR] vs 31.9 months; hazard ratio [HR], 0.56; 95% confidence interval [CI], 0.43-0.73; P<0.001) and achieved a >3-fold increase in minimal residual disease (MRD)-negativity rate (10-5 sensitivity threshold; 24.2% vs 7.3%; P<0.001) versus Rd alone in transplant-ineligible patients with NDMM (Facon T, N Engl J Med 2019). With longer follow-up (median follow-up, 56.2 months), D-Rd significantly improved overall survival (OS) versus Rd (HR, 0.68; 95% CI, 0.53-0.86; P=0.0013; Facon T, Lancet Oncol 2021). Here, we present an updated analysis of MAIA after a median follow-up of 64.5 months.
Methods: Patients with NDMM ineligible for high-dose chemotherapy and autologous stem cell transplant were randomized 1:1 to receive Rd ± DARA. Randomization was stratified by International Staging System disease stage (I vs II vs III), region (North America vs other), and age (<75 vs ≥75 years). All patients received 28-day cycles of Rd (R: 25 mg PO on Days 1-21; d: 40 mg PO on Days 1, 8, 15, and 22). In the D-Rd arm, DARA (16 mg/kg IV) was given once weekly in Cycles 1-2, once every 2 weeks in Cycles 3-6, and once every 4 weeks thereafter. In both treatment arms, patients were treated until disease progression or unacceptable toxicity. The primary endpoint was PFS. Key secondary endpoints included MRD-negativity rate (10-5 sensitivity, clonoSEQ® version 2.0), overall response rate (ORR), OS, and safety.
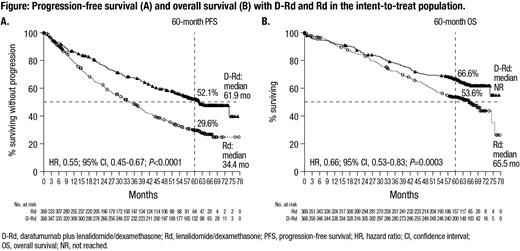
Results: In total, 737 patients were randomized (D-Rd, n=368; Rd, n=369). Baseline characteristics were well balanced between treatment arms. After a median follow-up of 64.5 months, PFS was improved with D-Rd versus Rd (median, 61.9 months vs 34.4 months; HR, 0.55; 95% CI, 0.45-0.67; P<0.0001; Figure A). A 34% reduction in the risk of death was observed with D-Rd versus Rd; median OS was NR with D-Rd versus 65.5 months with Rd (HR, 0.66; 95% CI, 0.53-0.83; P=0.0003). The estimated 60-month OS rate was 66.6% with D-Rd and 53.6% with Rd (Figure B). The ORR was higher for the D-Rd arm versus the Rd arm (92.9% vs 81.6%; P<0.0001). The MRD-negativity rate was also higher for D-Rd versus Rd (32.1% vs 11.1%; P<0.0001), as was the rate of sustained MRD negativity lasting ≥12 months (18.8% vs 4.1%; P<0.0001).
The most common (occurring in ≥15% of patients in either arm) grade 3/4 treatment-emergent adverse events (TEAEs; D-Rd/Rd) were neutropenia (54.1%/37.0%), anemia (17.0%/21.6%), pneumonia (19.5%/10.7%), and lymphopenia (16.5%/11.2%); grade 3/4 infection rates were 42.6%/29.6%. The most common serious TEAE in both arms was pneumonia (18.7%/10.7%). 14.6% of patients in the D-Rd arm and 23.8% of patients in the Rd arm discontinued treatment due to TEAEs.
Conclusions: In this updated analysis of MAIA, the addition of DARA to Rd continued to demonstrate PFS and OS benefits in transplant-ineligible patients with NDMM after a median follow-up of >5 years. D-Rd also achieved a nearly 3-fold higher MRD-negativity rate and a ≥4-fold higher ≥12-month sustained MRD-negativity rate versus Rd alone. No new safety concerns were observed with longer follow-up. These results continue to support the frontline use of D-Rd in transplant-ineligible patients with NDMM. Further updated OS results based on extended follow-up will be presented at the meeting.
Disclosures
Kumar:AbbVie,: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen,: Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda,: Membership on an entity's Board of Directors or advisory committees, Research Funding; Adaptive,: Membership on an entity's Board of Directors or advisory committees, Research Funding; KITE,: Research Funding; MedImmune/Astra Zeneca,: Membership on an entity's Board of Directors or advisory committees, Research Funding; Merck,: Research Funding; Novartis,: Research Funding; Roche: Research Funding; Sanofi: Research Funding; Oncopeptides: Other: Independent review committee. Moreau:AbbVie, Janssen, Celgene, Amgen, and Sanofi: Honoraria. Bahlis:Amgen: Consultancy, Honoraria; Genentech: Consultancy; Janssen: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria; GSK: Consultancy, Other; Forus: Consultancy, Honoraria; Takeda: Consultancy; Karyopharm Therapeutics: Consultancy, Honoraria; Celgene: Consultancy, Honoraria. Plesner:Janssen, Celgene, Takeda, Oncopeptides, Genentech, CSL Behring, AbbVie: Membership on an entity's Board of Directors or advisory committees; Janssen, Genmab, Celgene, Takeda, Oncopeptides, Genentech, AbbVie, Roche, Bristol Myers Squibb: Research Funding. Orlowski:Abbvie, BioTheryX, Inc., Bristol-Myers Squibb, Janssen Biotech, Karyopharm Therapeutics, Inc., Meridian Therapeutics, Monte Rosa Therapeutics, Neoleukin Corporation, Oncopeptides AB, Regeneron Pharmaceuticals, Inc., Sanofi-Aventis, and Takeda Pharmaceutic: Honoraria, Membership on an entity's Board of Directors or advisory committees; Asylia Therapeutics, Inc.: Current equity holder in private company; Asylia Therapeutics, Inc., BioTheryX, Inc., Heidelberg Pharma, Inc.: Research Funding; CARsgen Therapeutics, Celgene/Bristol Myers Squibb, Exelixis, Janssen Biotech, Sanofi-Aventis, Takeda Pharmaceuticals North America, Inc.: Research Funding. Basu:Sanofi, Pfizer, BMS: Consultancy, Speakers Bureau; Sanofi: Honoraria, Other: advisory board. Nahi:Genmab: Current Employment. Hulin:BMS: Honoraria; GSK: Honoraria; Takeda: Honoraria; Sanofi: Honoraria; Amgen: Honoraria; Janssen: Honoraria. Quach:AbbVie: Consultancy, Honoraria, Research Funding; GSK: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: leadership or fiduciary role, receipt of free drug for investigator-initiated study , Research Funding; Karyopharm: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: receipt of free drug for investigator-initiated study, Research Funding; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Antengene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi: Membership on an entity's Board of Directors or advisory committees, Other: receipt of free drug for investigator-initiated study, Research Funding; CSL: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: leadership or fiduciary role , Research Funding; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: leadership or fiduciary role, receipt of free drug for investigator-initiated study , Research Funding. Goldschmidt:Molecular Partners: Research Funding; Merck Sharp and Dohme (MSD): Research Funding; Mundipharma GmbH: Research Funding; Takeda: Research Funding; Novartis: Honoraria, Research Funding; Adaptive Biotechnology: Consultancy; GlaxoSmithKline (GSK): Honoraria; Amgen, BMS, Celgene, Chugai, Dietmar-Hopp-Foundation, Janssen, Johns Hopkins University, Sanofi: Other: Grants and/or provision of Investigational Medicinal Product; Amgen, BMS, Celgene, Chugai, Janssen, Incyte, Molecular Partners, Merck Sharp and Dohme, Sanofi, Mundipharma GmbH, Takeda, Novartis: Research Funding; Amgen, BMS, Janssen, Sanofi, Takeda: Membership on an entity's Board of Directors or advisory committees; SANOFI: Consultancy, Honoraria, Other: Grants, Research Funding; Incyte: Research Funding; Celgene: Consultancy, Honoraria, Other: Grants, Research Funding; Janssen: Consultancy, Honoraria, Other: Grants, Research Funding; Chugai: Honoraria, Other: grants, Research Funding; BMS: Consultancy, Honoraria, Other: Grants, Research Funding; AMGEN: Consultancy, Honoraria, Other: Grants, Research Funding; Amgen, BMS, Chugai, GlaxoSmithKline, Janssen, Novartis, Sanofi, Pfizer: Honoraria; Amgen, BMS, GlaxoSmithKline, Janssen, Novartis, Sanofi, Pfizer: Other: Support for attending meetings and/or travel; Array Biopharma: Research Funding; Dietmar-Hopp-Foundation: Research Funding. O'Dwyer:Janssen: Consultancy. Perrot:Abbvie: Honoraria; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees; GSK: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; Sanofi: Honoraria; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Venner:Janssen, BMS, Sanofi, FORUS, Pfizer, GSK, Amgen: Honoraria. Weisel:Stemline: Honoraria; Roche: Consultancy, Honoraria; Oncopeptides: Consultancy, Honoraria; Karyopharm: Consultancy, Honoraria; GSK: Consultancy, Honoraria; BeiGene: Consultancy, Honoraria; AstraZeneca: Honoraria; Adaptive Biotech: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria; BMS/Celgene: Consultancy, Honoraria, Research Funding; Novartis: Honoraria; Amgen: Consultancy, Honoraria, Research Funding. Raje:Celgene: Honoraria; Medscape: Honoraria; Amgen: Consultancy, Honoraria; Bristol Myers Squibb: Consultancy, Honoraria; Research to Practice: Honoraria; Two Seventy Bio: Research Funding; Massachusetts General Hospita: Current Employment; Janssen: Consultancy, Honoraria. Macro:Janssen: Honoraria, Other: Travel/accommodation, Research Funding; Takeda: Honoraria, Other: Travel/accommodation, Research Funding; GSK: Honoraria; Sanofi: Honoraria. Leleu:Amgen, Merck, BMS, GSK, Janssen, Oncopeptide, Takeda, Roche, Novartis, AbbVie, Sanofi, Gilead, Pfizer, Harpoon Therapeutic, Regeneron, Iteos: Consultancy, Honoraria; Pfizer: Honoraria; Amgen: Honoraria; Janssen: Honoraria; BMS: Honoraria; Takeda: Honoraria; Sanofi: Honoraria; Amgen, BMS/Celgene, Janssen, Takeda, Novartis, Sanofi, Merck, Oncopeptide, Karyopharm, Roche, Abbvie, Carsgen, GSK, and Harpoon Therapeutics: Honoraria. Pei:Janssen: Current Employment, Current equity holder in publicly-traded company. Krevvata:Janssen R& D, Johnson and Johnson: Current Employment. Carson:Janssen: Current Employment. Borgsten:Janssen: Current Employment. Usmani:Abbvie, Amgen, BMS, Celgene, EdoPharma, Genentech, Gilead, GSK, Janssen,Oncopeptides, Sanofi, Seattle Genetics, SecuraBio, SkylineDX, Takeda, TeneoBio: Consultancy; Amgen, BMS, Janssen, Sanofi: Speakers Bureau; Amgen, Array Biopharma, BMS, Celgene, GSK, Janssen, Merck, Pharmacyclics, Sanofi, Seattle Genetics, SkylineDX, Takeda: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal